Research and development of innovative procedures at the frontiers of knowledge regarding the computational modeling and simulation of the human cardiovascular system (CVS) and its application in the diagnosis, treatment and surgical planning of several diseases based on patient-specific data assimilation and medical images.
The arterial atherothrombotic cardiovascular disease (AACD), which main clinical manifestations are ischemic heart disease and stroke, is responsible nowadays in Brazil for twice the deaths of the second cause of death: the “Cancer” group. AACD is also considered the leading cause of disease-related retirements, the first cause of years of life lost due to incapability, the second leading cause of hospitalizations and the main cost of those (due to the use of highly complex procedures and their high cost). Because of those reasons, AACD represents a high priority for the health system. The inadequate control of these diseases indicates a lack of knowledge on cardiovascular health (including the development process of intra-arterial atherothrombotic plaques) and on the use of appropriate technologies for diagnosis and treatment. The computational modeling of the human CVS indicates that through these models, it is possible to achieve a better understanding of the process of atherothrombosis. In this sense, the use of imaging methods, such as angiography and intravascular ultrasound, would foster the improvement of computational modeling of the human CVS aiming at its use in the clinic.
Meanwhile, most of the cerebral hemorrhages are caused by the rupture of intracranial aneurysms, which are a pathologic dilation of cerebral arteries (frequently found close to the circle of Willis). Advanced medical imaging is able to detect undamaged aneurysms, for which specific measures should be taken in order to treat them appropriately (especially by the deployment of stents and other devices). Simulations that take into account the specific characteristics of the patient are necessary for planning and optimization of the device, which goal is to promote the thrombosis of the aneurysm. Detailed information of hemodynamic processes in cerebral arteries (such as those obtained by the simulations previously described) is also essential to understand the mechanisms responsible for the beginning and development of cerebrovascular diseases.
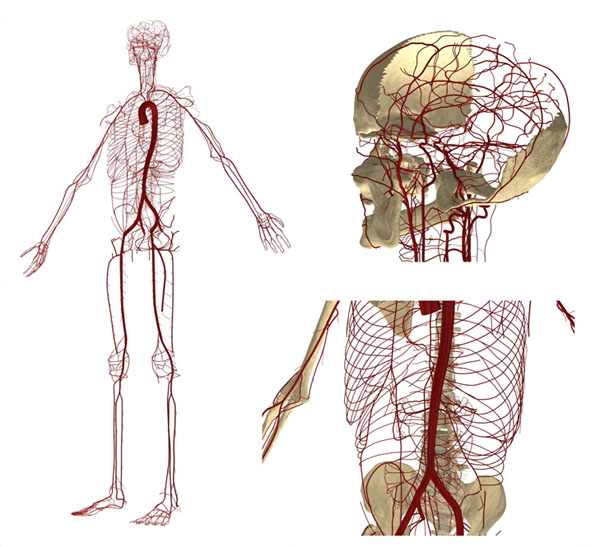 Figure 1. Anatomically Detailed Arterial Network Model (ADAN Model)
Figure 1. Anatomically Detailed Arterial Network Model (ADAN Model)
In the Associate Laboratory HeMoLab, a computational model called ADAN (Anatomically Detailed Arterial Network) was developed. This model incorporates all arteries acknowledged by the specialized literature (over 2000 arterial vessels) for a male patient with regular vascular anatomy. The geometry of the ADAN model was developed in the 3D space and enables to simulate the propagation of pulse pressure and blood flow distribution along the vascular territories of the human body (for more information, please visit: http://hemolab.lncc.br/adan-web). This model, shown in Figure 1, is the basis of the activities proposed below. Figure 2 presents the arterial pressure predicted by the ADAN model throughout the cardiac cycle.
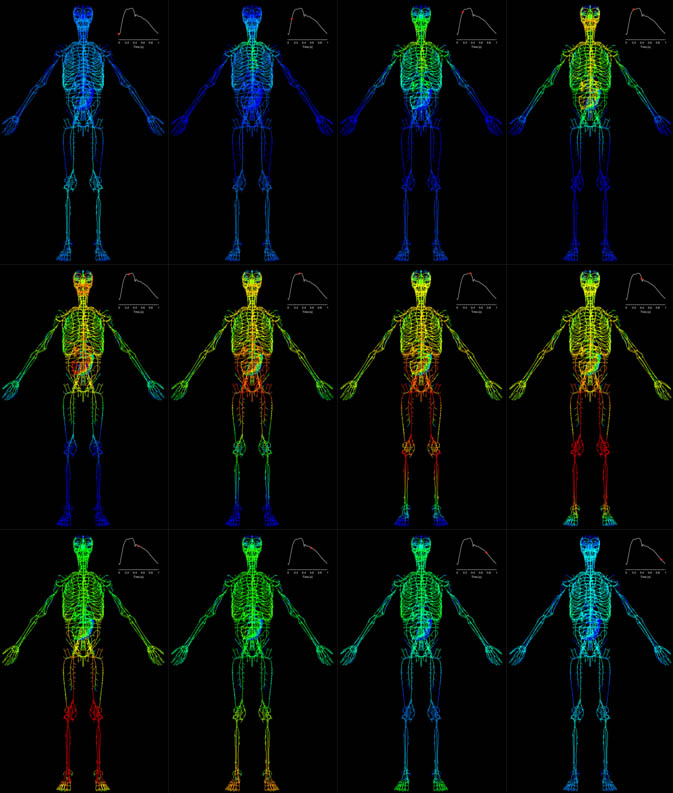 Figure 2. Arterial pressure given by the ADAN model throughout the cardiac cycle
Figure 2. Arterial pressure given by the ADAN model throughout the cardiac cycle
Activities
- Study and development of a detailed and anatomically accurate computational model of the venous part of the human CVS, regarding physiological and hemodynamic parameters (including the development of new and robust computational methods for their solution), Incorporation of the model in the ADAN-model (please check: http: //hemolab.lncc.br/adan-web).
- Study and development of advanced computational models to represent the heart chambers’ behavior (atria and ventricles) and its valves. Study of the human CVS hemodynamic behavior in normal and altered conditions (by diseases or medical procedures) including the extended ADAN model (i.e. incorporating the arterial, venous and pulmonary circulation).
- Study and development of advanced computational models for blood flow in peripheral arterial beds (level of arterioles, capillaries and venules).
- Study and development of advanced computational techniques to automatically build the topology and geometry of peripheral arterial beds capable of enabling (i) the incorporation of existing collateral circulation in these beds and (ii) the study of microcirculation in tissues and organs such as the heart, liver and kidney.
- To conduct the study and development of advanced computational models customized to the patient through data obtained by processing of computed tomography (CT) medical images: anatomically detailed and accurate from the point of view of physiological and hemodynamic coronary blood flow, including effects of existing collateral circulation in the myocardium so as to allow the noninvasive computational calculation of FFR (simply using the CT mode).
- To study and develop computational models to identify the mechanical properties of tissues through data obtained by processing of medical images from intravascular ultrasound (IVUS) and orthogonal angiography (AX). This will allow performing in-vivo computational histology and therefore, to develop hemodynamic indices associated with stability (rupture) of atheromatous plaques, supporting diagnosis and therapy.
- Study and development of the 1D+ model (i.e. variational Galerkin formulations with hybrid interpolation) in the modeling of blood flow in tubular domains, able to compete with the Navier-Stokes 3D models keeping the same degree of precision, while having a computational cost equivalent to the 1D model in order to speed up calculation times shear stress on arterial walls.
- Study and development of multi-scale models in computer simulation of the behavior of biological tissues. Applying multi-scale variational formulations, theory of solid mechanics for large deformations and sensitivity analysis to change parameters associated with the collagen, elastin and smooth muscle content of the vessels wall and the shape change of the arterial district, these models allow to simulate the emergence, evolution and instability (rupture) of aneurysms and/or atherosclerotic plaques.
- Modeling and computer simulation for in vivo characterization and quantification of (i) mechanical properties of biological tissues that form the arterial wall and (ii) the kinetics of certain regions of the heart muscle. The development of indices dedicated to support diagnosis and therapy is foreseen.
- To conduct the study and development of advanced computational models, patient-customized through data obtained by processing medical images from computed tomography (CT). These models are intended to be anatomically detailed and accurate from a physiological point of view and should be able to accurately describe the blood flow patterns in cerebral aneurysms, including effects of interaction between blood and arteries in order to allow noninvasive computational calculations of indicators associated to the stability (rupture) of aneurysms.
- Research and assessment of methods for automatic quantification of mechanical properties and the kinetics of heart muscle regions including the representation and visualization of directional information extracted from 3D dynamic multimodal sequences (CT, MRI, Ultrasound and Nuclear Medicine) through mathematical operators such as divergent, rotational and analysis of critical points.
- Study and development of computational tools to perform customized virtual experiments regarding implantation of endovascular devices for cerebral aneurysms. These tools will be used to study hemodynamic changes caused by choices made on the design of the device (stent).
- Development and implementation of a three-dimensional composite mesh generator taking into account the complex pattern of bifurcations of the arterial tree, the implanted devices inside arteries and all the necessary features to perform reliable finite elements simulations.
- Development and implementation of boundary conditions for terminal arteriesof the brain’s mathematical model (which must necessarily end after a number of generations away from the circle of Willis). It is important to highlight that usually there are hundreds of terminal arteries with unknown pressure at their outlets. This development is critical to foster the physiologically correct results in patient-specific simulations.
- Application of artificial intelligence methods for determining specific points in the ECG.
- One of the main difficulties faced by tissue engineering based on 3D bio-printers is associated with the life sustainability of the deposited (and generated) cells around the implanted structures (scaffolds). In fact, the absence of the vascular system to meet the required transport of oxygen and other nutrients and the removal of carbon dioxide and metabolic waste can determine the failure of the whole process. However, the proposed activities 1-8 may have a fundamental role in reducing this problem. This activity seeks then to train INCT-MACC’s staff to achieve the required knowledge and technology in tissue engineering to build customized patient-specific cardiac prostheses through 3D bio-printers based on computer modeling and simulation of physiological systems (including the automatic generation of arterial beds and its hemodynamic simulation).
Goals
- By the end of 2017: Development of the extended ADAN model with the incorporation of all the arteries and veins reported by the medical literature.
- By the end of 2019: Incorporation of the heart valves and chambers’ models to the extended ADAN model.
- By the end of 2021: Incorporation of patient-specific vascular beds models in the extended ADAN model in specific organs and tissues.
- By the of 2017: Simulation of the human CVS behavior in both normal and altered conditions (by disease or surgical intervention) in order to assist the diagnosis, treatment, prognosis of diseases and the planning of medical procedures.
- By the end of 2019: Development of a computational modelling system for the coronary circulation and establishment of robust computational indices to assess the functional severity of stenosis.
- By the end of 2019: Development of computational models for cerebral aneurysms and definition of robust computational indices for assessing the functional severity of aneurysms and their relative rupture’s risk.
- By the end of 2021: Computational simulation of several medical procedures such as angioplasty, implantation of stents, clips, anastomoses and bridges.
- By the end of 2021: Simulation of transport of drugs and other substances in the human CVS.
- By the end of 2021: Consolidation of the knowledge in developing cardiac prostheses using bio-printers (including the tissue vascularization).
- By the end of 2017: Development of a set of methods for quantification and visualization of cardiac motion through multimodal images.
- By the end of 2021: Making simulation tools generated within this specific goal available to the public as accessible and customizable services through web interfaces (Science gateways).
- By the end of 2019: Provision of one immune to artifacts algorithm for the detection of specific points in the ECG.
Impacts
- Better understanding of the hemodynamic phenomena associated with the cardiovascular condition of patients (both: normal conditions as well as in the case of abnormal conditions due to disease or human intervention)
- Better understanding of the onset and progress of cardiovascular diseases and their relation with blood circulation
- Possibility to analyze the postoperative hemodynamic effects and consequences in terms of waveforms and shear stress in arterial and venous vessels.
- Replacement of intravascular studies by computational methodologies for the calculation of the risk of myocardial infarction (as an alternative to FFR index) – Computational Evaluation of Fractional Flow Reserve (CE_FFR)
- Reduction of the technical complexity in assessing the risk of myocardial infarction.
- Reduction of operational costs associated to medical procedures and postoperative recovery.
- Better understanding of the underlying mechanisms of the pathological condition determined by cerebral aneurysms.
- Improvement in procedures for risk analysis of ruptured cerebral aneurysms.

|
|

